What types of isomerism are typical for arenes? Examples. Nomenclature and isomerism of arenes
Aromatic hydrocarbons– compounds of carbon and hydrogen, the molecule of which contains a benzene ring. The most important representatives of aromatic hydrocarbons are benzene and its homologues - products of the replacement of one or more hydrogen atoms in a benzene molecule with hydrocarbon residues.
The structure of the benzene molecule
The first aromatic compound, benzene, was discovered in 1825 by M. Faraday. Its molecular formula was established - C 6 H 6. If we compare its composition with the composition of a saturated hydrocarbon containing the same number of carbon atoms - hexane (C 6 H 14), then we can see that benzene contains eight less hydrogen atoms. As is known, the appearance of multiple bonds and cycles leads to a decrease in the number of hydrogen atoms in a hydrocarbon molecule. In 1865, F. Kekule proposed its structural formula as cyclohexanthriene - 1, 3, 5.

Thus, the molecule corresponding Kekule's formula, contains double bonds, therefore, benzene must be unsaturated, i.e., it must easily undergo addition reactions: hydrogenation, bromination, hydration, etc.
However, data from numerous experiments have shown that benzene enters into addition reactions only under harsh conditions (at high temperatures and lighting) and is resistant to oxidation. The most characteristic reactions for it are substitution reactions; therefore, benzene is closer in character to marginal hydrocarbons.
Trying to explain these discrepancies, many scientists have proposed various options for the structure of benzene. The structure of the benzene molecule was finally confirmed by the reaction of its formation from acetylene. In reality, the carbon-carbon bonds in benzene are equivalent, and their properties are not similar to those of either single or double bonds.
Currently, benzene is denoted either by the Kekule formula or by a hexagon in which a circle is depicted.
![]()
So what is special about the structure of benzene? Based on the researchers' data and calculations, it was concluded that all six carbon atoms are in a state sp 2 -hybridization and lie in the same plane. Unhybridized p-orbitals of carbon atoms that make up double bonds (Kekule formula) are perpendicular to the plane of the ring and parallel to each other.
They overlap each other, forming a single π-system. Thus, the system of alternating double bonds depicted in Kekulé’s formula is a cyclic system of conjugated, overlapping bonds. This system consists of two toroidal (donut-like) regions of electron density lying on either side of the benzene ring. Thus, it is more logical to depict benzene as a regular hexagon with a circle in the center (π-system) than as cyclohexatriene-1,3,5. 
The American scientist L. Pauling proposed to represent benzene in the form of two boundary structures that differ in the distribution of electron density and constantly transform into each other, i.e., consider it an intermediate compound, “averaging” of two structures.
Bond length measurements confirm these assumptions. It was found that all C-C bonds in benzene have the same length (0.139 nm). They are slightly shorter than single C-C bonds (0.154 nm) and longer than double bonds (0.132 nm).
There are also compounds whose molecules contain several cyclic structures.

Isomerism and nomenclature
Benzene homologues are characterized by isomerism of the position of several substituents. The simplest homolog of benzene - toluene (methylbenzene) - does not have such isomers; the following homologue is presented as four isomers:

The basis of the name of an aromatic hydrocarbon with small substituents is the word benzene. The atoms in the aromatic ring are numbered from highest to lowest substituent:

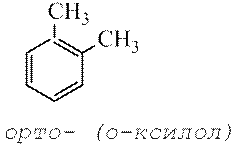
According to the old nomenclature, positions 2 and 6 are called orthopositions, 4 - pair-, and 3 and 5 - meta-provisions.
Physical properties
Under normal conditions, benzene and its simplest homologues are very toxic liquids with a characteristic unpleasant odor. They dissolve poorly in water, but well in organic solvents.
Chemical properties of benzene
Substitution reactions. Aromatic hydrocarbons undergo substitution reactions.
1. Bromination. When reacting with bromine in the presence of a catalyst, iron bromide (ΙΙΙ), one of the hydrogen atoms in the benzene ring can be replaced by a bromine atom:

2. Nitration of benzene and its homologues. When an aromatic hydrocarbon interacts with nitric acid in the presence of sulfuric acid (a mixture of sulfuric and nitric acids is called a nitrating mixture), the hydrogen atom is replaced by a nitro group -NO2:

By reducing the nitrobenzene formed in this reaction, aniline is obtained, a substance that is used to obtain aniline dyes:

This reaction is named after the Russian chemist Zinin.
Addition reactions. Aromatic compounds can also undergo addition reactions to the benzene ring. In this case, cyclohexane or its derivatives are formed.
1. Hydrogenation. Catalytic hydrogenation of benzene occurs at a higher temperature than the hydrogenation of alkenes:
![]()
2. Chlorination. The reaction occurs when illuminated with ultraviolet light and is free radical:

Benzene homologues
The composition of their molecules corresponds to the formula C n H 2 n-6. The closest homologues of benzene are:
All homologues of benzene following toluene have isomers. Isomerism can be associated both with the number and structure of the substituent (1, 2), and with the position of the substituent in the benzene ring (2, 3, 4). Compounds of the general formula C 8 H 10:

According to the old nomenclature used to indicate the relative location of two identical or different substituents on the benzene ring, the prefixes are used ortho- (abbreviated o-) - substituents are located at neighboring carbon atoms, meta-(m-) – through one carbon atom and pair— (P-) – substituents against each other.
The first members of the homologous series of benzene are liquids with a specific odor. They are lighter than water. They are good solvents.
Benzene homologues react substitutions ( bromination, nitration). Toluene is oxidized by permanganate when heated:

Benzene homologues are used as solvents to produce dyes, plant protection products, plastics, and medicines.



















Aromatic chemical compounds, or arenes, are a large group of carbocyclic compounds whose molecules contain a stable ring of six carbon atoms. It is called the “benzene ring” and is responsible for the special physical and chemical properties of arenes.
Aromatic hydrocarbons primarily include benzene and all its homologues and derivatives.
Arene molecules may contain several benzene rings. Such compounds are called polynuclear aromatic compounds. For example, naphthalene is a well-known drug for protecting woolen products from moths.
Benzene
This simplest representative of arenes consists only of a benzene ring. Its molecular formula is C 6 Η 6. The structural formula of the benzene molecule is most often represented by the cyclic form proposed by A. Kekule in 1865.
The advantage of this formula is that it accurately reflects the composition and equivalence of all C and H atoms in the ring. However, it could not explain many of the chemical properties of arenes, so the statement about the presence of three conjugated C=C double bonds is erroneous. This became known only with the advent of modern connection theory.
Meanwhile, today the formula for benzene is often written in the manner proposed by Kekule. Firstly, with its help it is convenient to write equations of chemical reactions. Secondly, modern chemists see in it only a symbol, and not a real structure. The structure of the benzene molecule is today conveyed by various types of structural formulas.
Structure of the benzene ring
The main feature of the benzene ring is the absence of single and double bonds in it in the traditional sense. In accordance with modern concepts, the benzene molecule appears as a flat hexagon with side lengths equal to 0.140 nm. It turns out that the length of the C-C bond in benzene is an intermediate value between single (its length is 0.154 nm) and double (0.134 nm). The C-H bonds also lie in the same plane, forming an angle of 120° with the edges of the hexagon.
Each C atom in the benzene structure is in the sp2 hybrid state. It is connected through its three hybrid orbitals with two C atoms located nearby and one H atom. That is, it forms three s-bonds. Another, but already unhybridized, 2p orbital overlaps with the same orbitals of neighboring C atoms (to the right and left). Its axis is perpendicular to the plane of the ring, which means that the orbitals overlap above and below it. In this case, a common closed π-electron system is formed. Due to the equal overlap of the 2p orbitals of the six C atoms, a kind of “equalization” of the C-C and C=C bonds occurs.
The result of this process is the similarity of such “one and a half” bonds with both double and single bonds. This explains the fact that arenes exhibit chemical properties characteristic of both alkanes and alkenes.
The energy of the carbon-carbon bond in the benzene ring is 490 kJ/mol. Which is also the average between the energies of a single and multiple double bond.

Arena nomenclature
The basis for the names of aromatic hydrocarbons is benzene. Atoms in the ring are numbered from the highest substituent. If the substituents are equivalent, then the numbering is carried out along the shortest path.
For many homologues of benzene, trivial names are often used: styrene, toluene, xylene, etc. To reflect the relative position of substituents, it is customary to use the prefixes ortho-, meta-, para-.
If the molecule contains functional groups, for example, carbonyl or carboxyl, then the arene molecule is considered as an aromatic radical connected to it. For example, -C 6 H 5 - phenyl, -C 6 H 4 - phenylene, C 6 H 5 -C H 2 - benzyl.
Physical properties
The first representatives in the homologous series of benzene are colorless liquids with a specific odor. Their weight is lighter than water, in which they are practically insoluble, but they dissolve well in most organic solvents.
All aromatic hydrocarbons burn with a smoky flame, which is explained by the high C content in the molecules. Their melting and boiling points increase with increasing molecular weights in the homologous series of benzene.
Chemical properties of benzene
Of the various chemical properties of arenes, substitution reactions should be mentioned separately. Also very significant are some addition reactions that occur under special conditions and oxidation processes.
Substitution reactions
Quite mobile π-electrons of the benzene ring are capable of reacting very actively with attacking electrophiles. This electrophilic substitution involves the benzene ring itself in benzene and the associated hydrocarbon chain in its homologues. The mechanism of this process has been studied in some detail by organic chemistry. The chemical properties of arenes associated with electrophile attack occur through three stages.
- First stage. The appearance of the π-complex is due to the binding of the π-electron system of the benzene ring to the X + particle, which binds to six π-electrons.

Bromination of benzene in the presence of iron or aluminum bromides without heating leads to the production of bromobenzene:
C 6 Η 6 + Br 2 —> C 6 Η 5 -Br + ΗBr.
Nitration with a mixture of nitric and sulfuric acids leads to the production of compounds with a nitro group in the ring:
C 6 Η 6 + ΗONO 2 -> C 6 Η 5 -NO 2 + Η 2 O.
Sulfonation is carried out by a bisulfonium ion formed as a result of the reaction:
3Η 2 SO 4 ⇄ SO 3 Η + + Η 3 O + + 2ΗSO 4 - ,
or sulfur trioxide.
The reaction corresponding to this chemical property of arenes is:
C 6 H 6 + SO 3 H + —> C 6 H 5 — SO 3 H + H + .
Alkyl and acyl substitution reactions, or Friedel-Crafts reactions, are carried out in the presence of anhydrous AlCl 3 .

These reactions are unlikely for benzene and occur with difficulty. The addition of hydrogen halides and water to benzene does not occur. However, at very high temperatures in the presence of platinum, a hydrogenation reaction is possible:
C 6 Η 6 + 3H 2 -> C 6 H 12.
When irradiated with ultraviolet light, chlorine molecules can join a benzene molecule:
C 6 Η 6 + 3Cl 2 —> C 6 Η 6 Cl 6 .
Oxidation reactions
Benzene is very resistant to oxidizing agents. Thus, it does not discolor the pink solution of potassium permanganate. However, in the presence of vanadium oxide, it can be oxidized by atmospheric oxygen to maleic acid:
C 6 H 6 + 4O -> COOΗ-CΗ = CΗ-COOΗ.
In air, benzene burns with the appearance of soot:
2C 6 Η 6 + 3O2 → 12C + 6 Η 2 O.
Chemical properties of arenes
- Substitution.


Orientation rules
Which position (o-, m- or p-) the substituent will occupy during the interaction of the electrophilic agent with the benzene ring is determined by the following rules:
- if there is already any substituent in the benzene ring, then it is this substituent that directs the incoming group to a certain position;
- all orienting substituents are divided into two groups: orientants of the first kind direct the incoming group of atoms to ortho- and para-positions (-NH 2, -OH, -CH 3, -C 2 H 5, halogens); orientants of the second kind direct the entering substituents to the meta position (-NO 2, -SO 3 H, -COHO, -COOH).
The orientations are listed here in order of decreasing directional force.
It is worth noting that this division of group substituents is conditional, due to the fact that in most reactions the formation of all three isomers is observed. Orientants only influence which of the isomers will be obtained in greater quantities.
Getting arenas
The main sources of arenes are dry distillation of coal and oil refining. Coal tar contains a huge amount of all kinds of aromatic hydrocarbons. Some types of oil contain up to 60% arenes, which can be easily isolated by simple distillation, pyrolysis or cracking.
Methods of synthetic preparation and chemical properties of arenes are often interrelated. Benzene, like its homologues, is obtained in one of the following ways.
1. Reforming of petroleum products. Dehydrogenation of alkanes is the most important industrial method for the synthesis of benzene and many of its homologues. The reaction is carried out by passing gases over a heated catalyst (Pt, Cr 2 O 3, Mo and V oxides) at t = 350-450 o C:
C 6 H 14 —> C 6 Η 6 + 4 Η 2.
2. Wurtz-Fittig reaction. It is carried out through the stage of obtaining organometallic compounds. As a result of the reaction, several products can be obtained.
3. Trimerization of acetylene. Acetylene itself, like its homologues, is capable of forming arenes when heated with a catalyst:
3C 2 Η 2 -> C 6 Η 6.
4. Friedel-Crafts reaction. The method of obtaining and converting benzene homologues has already been discussed above in the chemical properties of arenes.
5. Preparation from the corresponding salts. Benzene can be isolated by distilling benzoic acid salts with alkali:
C 6 Η 5 —COONa + NaOΗ —> C 6 Η 6 + Na 2 CO 3 .
6. Reduction of ketones:
C 6 Η 5 -CO-CΗ 3 + Zn + 2ΗCl -> C 6 Η 5 -CΗ 2 -CΗ 3 + Η 2 O + ZnCl 2 ;
CΗ 3 -C 6 Η 5 -CO-CΗ 3 + NΗ 2 -NΗ 2 —> CΗ 3 -C 6 Η 5 -CΗ 2 -CΗ 3 + Η 2 O.
Application of arenas
The chemical properties and areas of application of arenes are directly related, since the bulk of aromatic compounds are used for further synthesis in chemical production, and are not used in finished form. The exception is substances used as solvents.
Benzene C 6 × 6 is used mainly in the synthesis of ethylbenzene, cumene and cyclohexane. On its basis, intermediate products are obtained for the production of various polymers: rubbers, plastics, fibers, dyes, surfactants, insecticides, and medicines.

Toluene C 6 H 5 -CH 3 is used in the production of dyes, medicines and explosives.
Xylenes C 6 Η 4 (C 3) 2 in mixed form (technical xylene) are used as a solvent or starting preparation for the synthesis of organic substances.
Isopropylbenzene (or cumene) C 6 H 4 -C H (C H 3) 2 is the starting reagent for the synthesis of phenol and acetone.
Vinylbenzene (styrene) C 6 Η 5 -CΗ=CΗ 2 is the raw material for the production of the most important polymer material - polystyrene.
General consideration.
Aromatic hydrocarbons (arenes) are substances whose molecules contain one or more benzene rings - cyclic groups of carbon atoms with a special nature of bonds.
The concept of “benzene ring” immediately requires decoding. To do this, it is necessary to at least briefly consider the structure of the benzene molecule. The first structure of benzene was proposed in 1865 by the German scientist A. Kekule:
This formula correctly reflects the equivalence of six carbon atoms, but does not explain a number of special properties of benzene. For example, despite being unsaturated, benzene does not show a tendency to addition reactions: it does not discolor bromine water and a solution of potassium permanganate, i.e. does not give qualitative reactions typical for unsaturated compounds.
The structural features and properties of benzene were fully explained only after the development of the modern quantum mechanical theory of chemical bonds. According to modern concepts, all six carbon atoms in the benzene molecule are in the -hybrid state. Each carbon atom forms -bonds with two other carbon atoms and one hydrogen atom, lying in the same plane. The bond angles between the three -bonds are 120°. Thus, all six carbon atoms lie in the same plane, forming a regular hexagon (the skeleton of a benzene molecule).
Each carbon atom has one unhybridized p orbital.
Six such orbitals are located perpendicular to the flat -skeleton and parallel to each other (Fig. 21.1, a). All six p-electrons interact with each other, forming -bonds that are not localized in pairs, as in the formation of ordinary double bonds, but are combined into a single -electron cloud. Thus, circular conjugation occurs in the benzene molecule (see § 19). The highest -electron density in this conjugated system is located above and below the -skeleton plane (Fig. 21.1, b).

Rice. 21.1. The structure of the benzene molecule
As a result, all bonds between carbon atoms in benzene are aligned and have a length of 0.139 nm. This value is intermediate between the length of a single bond in alkanes (0.154 nm) and the length of a double bond in alkenes (0.133 nm). The equivalence of connections is usually depicted with a circle inside the cycle (Fig. 21.1, c). Circular conjugation gives an energy gain of 150 kJ/mol. This value constitutes the conjugation energy - the amount of energy that must be expended to disrupt the aromatic system of benzene (compare - the conjugation energy in butadiene is only 12 kJ/mol).
This electronic structure explains all the features of benzene. In particular, it is clear why benzene is difficult to enter into addition reactions - this would lead to a violation of conjugation. Such reactions are only possible under very harsh conditions.
Nomenclature and isomerism.
Conventionally, the arenas can be divided into two rows. The first includes benzene derivatives (for example, toluene or biphenyl), the second includes condensed (polynuclear) arenes (the simplest of them is naphthalene):

We will consider only the homologous series of benzene with the general formula.
Structural isomerism in the homologous series of benzene is due to the mutual arrangement of substituents in the nucleus. Monosubstituted benzene derivatives do not have positional isomers, since all atoms in the benzene ring are equivalent. Disubstituted derivatives exist in the form of three isomers, differing in the relative arrangement of substituents. The position of the substituents is indicated by numbers or prefixes:

The radicals of aromatic hydrocarbons are called aryl radicals. The radical is called phenyl.
Physical properties.
The first members of the homologous series of benzene (for example, toluene, ethylbenzene, etc.) are colorless liquids with a specific odor. They are lighter than water and insoluble in water. They dissolve well in organic solvents. Benzene and its homologues are themselves good solvents for many organic substances. All arenas burn with a smoky flame due to the high carbon content in their molecules.
Methods of obtaining.
1. Preparation from aliphatic hydrocarbons. When straight-chain alkanes with at least 6 carbon atoms per molecule are passed over heated platinum or chromium oxide, dehydrocyclization occurs - the formation of an arene with the release of hydrogen:
2. Dehydrogenation of cycloalkanes. The reaction occurs by passing vapors of cyclohexane and its homologues over heated platinum:

3. Preparation of benzene by trimerization of acetylene - see § 20.
4. Obtaining benzene homologues using the Friedel-Crafts reaction - see below.
5. Fusion of salts of aromatic acids with alkali:
Chemical properties.
General consideration. Possessing a mobile six -electrons, the aromatic nucleus is a convenient object for attack by electrophilic reagents. This is also facilitated by the spatial arrangement of the electron cloud on both sides of the flat skeleton of the molecule (Fig. 21.1, b)
The most typical reactions for arenes are those that proceed through the mechanism of electrophilic substitution, denoted by the symbol (from the English substitution electrophilic).
The mechanism of electrophilic substitution can be represented as follows. The electrophilic reagent XY (X is an electrophile) attacks the electron cloud, and due to weak electrostatic interaction, an unstable -complex is formed. The aromatic system is not yet disrupted. This stage proceeds quickly. In the second, slower stage, a covalent bond is formed between electrophile X and one of the carbon atoms of the ring due to two -electrons of the ring. This carbon atom goes from the -hybrid state. In this case, the aroma of the system is disrupted. The four remaining -electrons are shared among five other carbon atoms, and the benzene molecule forms a carbocation, or -complex.

Disruption of aromaticity is energetically unfavorable, therefore the structure of the β-complex is less stable than the aromatic structure. To restore aromaticity, a proton is removed from the carbon atom bound to the electrophile (third stage). In this case, two electrons return to the -system, and thereby aromaticity is restored:

Electrophilic substitution reactions are widely used for the synthesis of many benzene derivatives.
Chemical properties of benzene.
1. Halogenation. Benzene does not react with chlorine or bromine under normal conditions. The reaction can only take place in the presence of anhydrous catalysts. As a result of the reaction, halogen-substituted arenes are formed:

The role of the catalyst is to polarize a neutral halogen molecule to form an electrophilic particle from it:
2. Nitration. Benzene reacts very slowly with concentrated nitric acid even when heated. However, under the action of the so-called nitrating mixture (a mixture of concentrated nitric and sulfuric acids), the nitration reaction occurs quite easily:
3. Sulfonation. The reaction easily takes place under the influence of “fuming” sulfuric acid (oleum):
4. Friedel-Crafts alkylation. As a result of the reaction, an alkyl group is introduced into the benzene ring to produce benzene homologues. The reaction occurs when benzene is exposed to haloalkanes in the presence of catalysts - aluminum halides. The role of the catalyst is reduced to the polarization of the molecule with the formation of an electrophilic particle:
Depending on the structure of the radical in the haloalkane, different benzene homologues can be obtained:
5. Alkylation with alkenes. These reactions are widely used industrially to produce ethylbenzene and isopropylbenzene (cumene). Alkylation is carried out in the presence of a catalyst. The reaction mechanism is similar to the mechanism of the previous reaction:
All reactions discussed above proceed through the mechanism of electrophilic substitution.
Reactions of addition to arenes lead to the destruction of the aromatic system and require large amounts of energy, so they occur only under harsh conditions.
6. Hydrogenation. The reaction of hydrogen addition to arenes occurs under heating and high pressure in the presence of metal catalysts (Ni, Pt, Pd). Benzene is converted into cyclohexane, and benzene homologues are converted into cyclohexane derivatives:

7. Radical halogenation. The interaction of benzene vapor with chlorine occurs via a radical mechanism only under the influence of hard ultraviolet radiation. In this case, benzene adds three chlorine molecules and forms a solid product - hexachlorocyclohexane:

8. Oxidation by air oxygen. In terms of its resistance to oxidizing agents, benzene resembles alkanes. Only with strong heating (400 °C) of benzene vapor with atmospheric oxygen in the presence of a catalyst, a mixture of maleic acid and its anhydride is obtained:

Chemical properties of benzene homologues.
Benzene homologues have a number of special chemical properties associated with the mutual influence of the alkyl radical on the benzene ring, and vice versa.
Side chain reactions. The chemical properties of alkyl radicals are similar to alkanes. The hydrogen atoms in them are replaced by halogen by a free radical mechanism. Therefore, in the absence of a catalyst, upon heating or UV irradiation, a radical substitution reaction occurs in the side chain. The influence of the benzene ring on alkyl substituents leads to the fact that the hydrogen atom at the carbon atom directly bonded to the benzene ring (a-carbon atom) is always replaced.

Substitution in the benzene ring is possible only by the mechanism in the presence of a catalyst:
Below you will find out which of the three isomers of chlorotoluene are formed in this reaction.
When benzene homologues are exposed to potassium permanganate and other strong oxidizing agents, the side chains are oxidized. No matter how complex the chain of the substituent is, it is destroyed, with the exception of the -carbon atom, which is oxidized into a carboxyl group.
Homologues of benzene with one side chain give benzoic acid:

Rules for orientation (substitution) in the benzene ring.
The most important factor determining the chemical properties of a molecule is the distribution of electron density in it. The nature of the distribution depends on the mutual influence of the atoms.
In molecules that have only -bonds, the mutual influence of atoms occurs through the inductive effect (see § 17). In molecules that are conjugated systems, the mesomeric effect manifests itself.
The influence of substituents transmitted through a conjugated system of -bonds is called the mesomeric (M) effect.
In a benzene molecule, the electron cloud is distributed evenly over all carbon atoms due to conjugation.
If any substituent is introduced into the benzene ring, this uniform distribution is disrupted and a redistribution of the electron density in the ring occurs. The place where the second substituent enters the benzene ring is determined by the nature of the existing substituent.
Substituents are divided into two groups depending on the effect they exhibit (mesomeric or inductive): electron-supporting and electron-withdrawing.
Electron-donating substituents have an effect and increase the electron density in the conjugated system. These include the hydroxyl group -OH and the amino group. The lone pair of electrons in these groups enters into common conjugation with the -electronic system of the benzene ring and increases the length of the conjugated system. As a result, the electron density is concentrated in the ortho and para positions:

Alkyl groups cannot participate in general conjugation, but they exhibit an effect under which a similar redistribution of electron density occurs.
Electron-withdrawing substituents exhibit an -M effect and reduce the electron density in the conjugated system. These include the nitro group, sulfo group, aldehyde -CHO and carboxyl -COOH groups. These substituents form a common conjugated system with the benzene ring, but the overall electron cloud shifts towards these groups. Thus, the total electron density in the ring decreases, and it decreases least at the meta positions:
For example, toluene containing a substituent of the first kind is nitrated and brominated in para- and ortho-positions:

Nitrobenzene containing a substituent of the second type is nitrated and brominated in the meta position:

In addition to the orienting effect, substituents also influence the reactivity of the benzene ring: orientants of the 1st kind (except halogens) facilitate the entry of the second substituent; Orientants of the 2nd kind (and halogens) make it difficult.
ARENES (aromatic hydrocarbons)
Arenes or aromatic hydrocarbons – These are compounds whose molecules contain stable cyclic groups of atoms (benzene nuclei) with a closed system of conjugated bonds.
Why "Aromatic"? Because Some of a number of substances have a pleasant odor. However, nowadays the concept of “aromaticity” has a completely different meaning.
The aromaticity of a molecule means its increased stability, due to the delocalization of π-electrons in the cyclic system.
Arene aromaticity criteria:
- Carbon atoms in sp 2 -hybridized state form a cycle.
- The carbon atoms are arranged in one plane(the cycle has a flat structure).
A closed system of conjugate connections contains
4n+2π electrons ( n– integer).
The benzene molecule fully meets these criteria. C 6 H 6.
Concept “ benzene ring” requires decryption. To do this, it is necessary to consider the structure of the benzene molecule.
IN
All bonds between carbon atoms in benzene are identical (there are no double or single bonds as such) and have a length of 0.139 nm. This value is intermediate between the length of a single bond in alkanes (0.154 nm) and the length of a double bond in alkenes (0.133 nm).The equivalence of connections is usually represented by a circle inside a cycle
Circular conjugation gives an energy gain of 150 kJ/mol. This value is conjugation energy is the amount of energy that must be expended to disrupt the aromatic system of benzene.
General formula: CnH2n-6(n ≥ 6)
Homologous series:
Benzene homologues are compounds formed by replacing one or more hydrogen atoms in a benzene molecule with hydrocarbon radicals (R):
ortho- (O-)
substituents on neighboring carbon atoms of the ring, i.e. 1,2-;
meta- (m-)
substituents through one carbon atom (1,3-);
pair- (P-)
substituents on opposite sides of the ring (1,4-).
aryl
C 6 H 5- (phenyl) And C6H
Aromatic monovalent radicals have the common name " aryl". Of these, two are the most common in the nomenclature of organic compounds:C 6 H 5- (phenyl) And C6H5CH2- (benzyl). 5 CH 2- (benzyl).
Isomerism:
structural:
1) positions of substituents for di-, three- And tetra-substituted benzenes (for example, O-, m- And P-xylenes);
2) carbon skeleton in the side chain containing at least 3 carbon atoms:
3) isomerism of R substituents, starting with R = C 2 H 5.
Chemical properties:
For arenes, reactions proceeding with preservation of the aromatic system, namely, substitution reactions hydrogen atoms associated with the ring.
2. Nitration
Benzene reacts with a nitrating mixture (a mixture of concentrated nitric and sulfuric acids):
3. Alkylation
Replacement of a hydrogen atom in the benzene ring with an alkyl group ( alkylation) occurs under the influence alkyl halides or alkenes in the presence of catalysts AlCl 3, AlBr 3, FeCl 3.
Substitution in alkylbenzenes:
Benzene homologs (alkylbenzenes) undergo substitution reactions more actively than benzene.
For example, during the nitration of toluene C 6 H 5 CH 3 Substitution of not one, but three hydrogen atoms can occur with the formation of 2,4,6-trinitrotoluene:
and facilitates substitution in these positions.On the other hand, under the influence of the benzene ring, the methyl group CH 3 in toluene it becomes more active in oxidation and radical substitution reactions compared to methane CH 4.
Toluene, unlike methane, oxidizes under mild conditions (discolors an acidified solution of KMnO 4 when heated):
Radical substitution reactions occur more easily than in alkanes. side chain alkylbenzenes:
This is explained by the fact that at the limiting stage, stable intermediate radicals are easily formed (at a low activation energy). For example, in case toluene a radical is formed benzyl Ċ H 2 -C 6 H 5 . It is more stable than alkyl free radicals ( Ċ N 3, Ċ H 2 R), because its unpaired electron is delocalized due to interaction with the π-electron system of the benzene ring:
Orientation rules
- The substituents present on the benzene ring direct the newly introduced group to certain positions, i.e. have an orienting effect.
According to their directing action, all substituents are divided into two groups:orientants of the first kind And orientants of the second kind.
Orientants of the 1st kind(ortho-para-orientators) direct subsequent substitution predominantly toortho- And pair- provisions.
These include electron donor groups (electronic effects of groups are indicated in brackets):
R ( +I); - OH(+M,-I); - OR(+M,-I); - NH 2(+M,-I); - NR 2(+M,-I) The +M effect is stronger than the -I effect in these groups.
Orientants of the 1st kind increase the electron density in the benzene ring, especially on the carbon atoms inortho- And pair-positions, which favors the interaction of these particular atoms with electrophilic reagents.Orientants of the 1st kind, increasing the electron density in the benzene ring, increase its activity in electrophilic substitution reactions compared to unsubstituted benzene.
A special place among the 1st kind orientants is occupied by halogens, which exhibitelectron-withdrawing properties:
-F (+M<–I ), -Cl (+M<–I ), -Br (+M<–I ).
Being ortho-para-orientants, they slow down electrophilic substitution. Reason - strong –I-the effect of electronegative halogen atoms, which reduces the electron density in the ring.
Orientants of the 2nd kind ( meta-orientators) direct subsequent substitution predominantly to meta-position.
These include electron-withdrawing groups:
-NO 2 (–M, –I); -COOH (–M, –I); -CH=O (–M, –I); -SO3H (–I); -NH3+ (–I); -CCl 3 (–I).
Orientants of the 2nd kind reduce the electron density in the benzene ring, especially in ortho- And pair- provisions. Therefore, the electrophile attacks carbon atoms not in these positions, but in meta-position where the electron density is slightly higher.
Example:
Thus, the ease of electrophilic substitution for the compounds (given as examples) decreases in the order:
toluene C 6 H 5 CH Unlike benzene, its homologues are oxidized quite easily.ARENES
Aromatic hydrocarbons (arenes) – cyclic hydrocarbons, united by the concept of aromaticity, which determines common characteristics in structure and chemical properties.
Classification
Based on the number of benzene rings in the molecule, arenes are divided into on the:
mononuclear
multi-core

Nomenclature and isomerism
The structural ancestor of benzene series hydrocarbons is benzene C 6 H 6 from which the systematic names of homologues are derived.
For monocyclic compounds, the following non-systematic (trivial) names are retained:

The position of the substituents is indicated in the smallest numbers (the direction of numbering does not matter),
|
|
|
and for di-substituted compounds you can use the notation ortho, meta, pair.
|
|
|
|
If there are three substituents in the ring, they should receive the lowest numbers, i.e. the row “1,2,4” has an advantage over “1,3,4”.


1,2-dimethyl-4-ethylbenzene (correct name) 3,4-dimethyl-1-ethylbenzene (incorrect name)
The isomerism of monosubstituted arenes is due to the structure of the carbon skeleton of the substituent; in di- and polysubstituted benzene homologues, additional isomerism is added, caused by the different arrangement of substituents in the nucleus.
Isomerism of aromatic hydrocarbons with the composition C 9 H 12:
|
|
|
|
|
Physical properties
The boiling and melting points of arenes are higher than those of alkanes, alkenes, alkynes, they are slightly polar, insoluble in water and highly soluble in non-polar organic solvents. Arenas are liquids or solids that have specific odors. Benzenes and many condensed arenes are toxic, some of them exhibit carcinogenic properties. Intermediate products of the oxidation of condensed arenes in the body are epoxides, which either themselves directly cause cancer or are precursors of carcinogens.
Getting arenas
Many aromatic hydrocarbons are of great practical importance and are produced on a large industrial scale. A number of industrial methods are based on the processing of coal and oil.
Oil consists mainly of aliphatic and alicyclic hydrocarbons; to convert aliphatic or acyclic hydrocarbons into aromatic ones, methods for aromatizing oil have been developed, the chemical basis of which was developed by N.D. Zelinsky, B.A. Kazansky.
1. Cyclization and dehydrogenation:
2. Hydrodesmethylation:

3. Benzene homologues are prepared by alkylation or acylation followed by reduction of the carbonyl group.
a) Friedel-Crafts alkylation:

b) Friedel-Crafts acylation:

4. Preparation of biphenyl by the Wurtz-Fitting reaction:
5. Preparation of diphenylmethane by the Friedel-Crafts reaction:
Structure and chemical properties.
Aromaticity criteria:
Based on theoretical calculations and experimental studies of cyclic conjugated systems, it was found that a compound is aromatic if it has:
- Flat cyclic σ-skeleton;
- A conjugated closed π-electron system, covering all atoms of the ring and containing 4n + 2, where n = 0, 1, 2, 3, etc. This formulation is known as Hückel's rule. Aromaticity criteria allow one to distinguish conjugated aromatic systems from all others. Benzene contains a sextet of π electrons and follows Hückel's rule at n = 1.

What does aromaticity give:
Despite the high degree of unsaturation, aromatic compounds are resistant to oxidizing agents and temperature, and they are more prone to undergo substitution reactions rather than addition reactions. These compounds have increased thermodynamic stability, which is ensured by the high conjugation energy of the aromatic ring system (150 kJ/mol); therefore, arenes preferably enter into substitution reactions, as a result of which they retain aromaticity.
Mechanism of electrophilic substitution reactions in the aromatic ring:
The electron density of the π-conjugated system of the benzene ring is a convenient target for attack by electrophilic reagents.
Typically, electrophilic reagents are generated during a reaction using catalysts and appropriate conditions.
E – Y → E δ + – Y δ - → E + + Y -
Formation of a π-complex. The initial attack by the electrophile of the π-electron cloud of the ring leads to coordination of the reagent with the π-system and the formation of a donor-acceptor type complex called π-complex. The aroma system is not disrupted:

Formation of the σ-complex. The limiting stage, in which the electrophile forms a covalent bond with a carbon atom due to two electrons of the π-system of the ring, which is accompanied by the transition of this carbon atom from sp 2 - V sp 3 - hybrid state and aromatic disruption, the molecule turns into a carbocation.

Stabilization of the σ-complex. It is carried out by abstraction of a proton from the σ-complex using a base. In this case, due to the two electrons of the breaking covalent bond C–H, the closed π-system of the ring is restored, i.e. the molecule returns to the aromatic state:

Effect of substituents on reactivity and orientation of electrophilic substitution
Substituents on the benzene ring disrupt the distribution uniformity π- electron cloud of the ring and thereby influence the reactivity of the ring.
- Electron-donating substituents (D) increase the electron density of the ring and increase the rate of electrophilic substitution; such substituents are called activating.
- Electron-withdrawing substituents (A) reduce the electron density of the ring and reduce the reaction rate, called decontaminating.

 Entrance
Entrance