Nitration of benzene. Pyrotechnic chemistry: Chemistry and technology of high explosives - Orlova E.Yu Preparation through diazo compound
It is not possible to introduce more than three nitro groups into benzene and toluene molecules by nitration.
Amino-2-nitrotoluene l-Nitro-p-toluidine sn L h/ 1 mn 0, and Toluene Nitration- reduction with soda sulphide
M. S. Bykhovskaya describes a method for the separate determination of benzene and toluene. By nitration, benzene is converted into dinitrobenzene, and toluene into trinitrotoluene 77
When fractionating the liquid part of the catalyzate, 14.5 g of hydrocarbon per bp was isolated. -IG (756 mai) n 1.4967 df 0.8665. A comparison of the constants of this fraction with literature data for toluene shows that it consists of toluene.
Nitration of this fraction yielded a nitro product with mp. 69° C. A mixed melting test with 2,4-dinitrotoluene did not produce depression.
Xylyl production. As we saw from the works of Martinsen, with the introduction of the 2nd methyl group into the nucleus, the rate of the nitration reaction increases significantly. The speed of the nitration reaction of ti-xi-lol is several times greater than that of toluene.
Therefore, mixtures for nitration of xylene can be much weaker than for nitration of toluene. This is also the reason why xylene can be nitrated into trinitroxylene even under factory conditions. one phase, while for toluene nitration V one phase has never been produced anywhere, due to the fact that this is associated with a huge consumption of acids, and therefore uneconomical, not to mention other difficulties and disadvantages of nitration of toluene into trinitrotoluene in one phase.
Formation of significant amounts of zheta-substituted products when alkylation of toluene and other monosubstituted benzenes can be explained by the high reactivity of the attacking reagent. Since bromination is an example of a fairly mild substitution reaction, in this case there are strong differences between benzene and toluene, as well as between the meta and para positions in toluene.
Nitration is less selective than bromination; isopropylation is much less selective than nitration. At alkylation of toluene 30% of the summer isomer is formed. Moreover, with this reaction the differences between toluene and benzene become negligible.
A review of substitution reactions in the aromatic series allows us to draw a parallel between the selectivity of reactions with benzene and toluene, on the one hand, and between the meta- and para-positions in toluene, on the other. In both cases, selectivity decreases with increasing reaction ability of attacking agent 2. Table data. 4 illustrate these points.70
When considering the methods for determining toluene given in the literature, it seemed possible to use the photometric method. The latter has found wide application for the determination of small amounts of toluene, in particular in wastewater and in the air of industrial enterprises. Many colorimetric determinations of toluene are based on nitration and subsequent interaction of the resulting nitro compound with alkali or ammonia in various solvents: acetone, alcohol, methyl ethyl ketone, butanol, alcohol-ether mixture and others.
Depending on the intensity of cooling and stirring, the addition of toluene lasts 4-8 hours. The nitration temperature is maintained at about 50° and after the pouring is completed, it is heated for another period. % hour at 80-90°. Longer and stronger heating is impractical, since after the end of the infusion toluene nitration unlikely to continue.
For example, when heating is continued at 90° for 2 hours. The solidification temperature of dinitrotoluene increased in one case from 33.7° to only 35.6°. Upon completion of nitration, the contents of the apparatus are cooled to a temperature slightly higher than the temperature. melting of the dinitro product, and press the contents of the reactor using compressed air into one of the leaded separators (1,2), mixing the reaction mixture with such an amount of water that the dilution reaches approximately 16%. In this case, at first there is a very strong release of nitrogen oxides, for the removal of which a sufficiently powerful exhaust pipe is required. After settling for several hours at a temperature slightly higher than the melting point of the nitro product, the acid separates from the product forming the upper layer.377
Categories
Select heading 1. PHYSICAL AND CHEMICAL PROPERTIES OF OIL AND NATURAL GAS 3. BASICS OF OIL FIELDS DEVELOPMENT AND OPERATION 3.1. Flowing operation of oil wells 3.4. Operation of wells by submersible electric centrifugal 3.6. Concept of development of oil and gas wells 7. METHODS OF INFLUENCE ON THE NEAR-BOREBOTH ZONE OF THE FORMATION MAIN COMPONENTS OF THE FORMATION TESTER SCREW DOWNHOUSE MOTORS EMERGENCY AND SPECIAL OPERATING MODES OF ELECTRICAL EQUIPMENT UNITS FOR REPAIR AND DRILLING WELLS ANALYSIS OF CAUSES MA WELL PRODUCTIVITY ANALYSIS OF TECHNOLOGIES FOR CAPITAL REPAIRS OF WELLS Wellhead fittings ASPHALT RESIN-PARAFIN DEPOSITS Without headings SMOKELESS GAS COMBUSTION RODLESS WELL PUMPING UNITS blogun CIRCULATION SYSTEMS UNITS. combating hydrates COMBATING PARAFFIN DEPOSITION IN LIFTING PIPES drilling Drilling sidetracks DRILLING DIRECTIVE AND HORIZONTAL WELLS Drilling wells DRILL STRING DRILLING AUTOMATIC STATIONARY TONGS DRILLING UNITS AND INSTALLATIONS FOR GEOLOGICAL EXPLORATION DRILL ENIA DRILLING RIGS DRILLING PUMPS DRILLING PUMPS DRILLING HOSES DRILLING RIG IN PERMAFROST (MMP) VALVE. TYPES OF HETEROGENEOUSITY IN THE STRUCTURE OF OIL RESERVES Types of wells SCREW SUBMERSIBLE PUMPS DRIVEN AT THE WELLHEAD MOISTURE CONTENT AND HYDRATES OF NATURAL GAS COMPOSITION HYDRATE Impact various factors on the characteristics of the SDM QUESTIONS OF OPTIMIZATION OF THE OPERATION OF THE RESERVOIR - ESP SYSTEM SELECTION OF EQUIPMENT AND OPERATING MODE OF ESP SELECTION OF A POWER MACHINE Gas lift installation LN Gas lift operation of oil wells Gas lift method of oil production GASES OF OIL AND GAS FIELDS AND THEIR PROPERTIES GUIDE RATE FORMATION IN GAS CONDENSATE WELLS HYDRATE FORMATION IN OIL COLLECTION SYSTEM hydraulic protection of submersible electric motor GIDROKEY GKSH-1500MT hydraulic piston pump Chapter 8. MEANS AND METHODS OF CALIVATION AND CHECKING FLOW MEASURING SYSTEMS DEEP PUMPS Horizontal drilling MINING GEOLOGICAL CONDITIONS OF DRILLING OIL AND GAS WELLS GRANULOMETRIC (FELLOWS) NICH) COMPOSITION OF ROCK LONG LONG TRANSPORT OF OIL AND GAS DEFORMATION PRESSURE GAUGES Diaphragm electric pumps DIESEL-HYDRAULIC UNIT SAT-450 DIESEL AND DIESEL-HYDRAULIC UNITS DYNAMOMETERING OF BLOOM UNITS WITH LMP CONSTRUCTIONS OF OJSC "ORENBURGNEFT" oil production oil production in difficult conditions OIL PRODUCTION USING SPU LIQUID PRESSURE GAUGES DOWNHEATH MOTORS Injection of acid solutions into the well ZAP ORIGINAL FIXTURES. PROTECTION OF OIL FIELD EQUIPMENT AGAINST CORROSION PROTECTION AGAINST CORROSION OF OIL FIELD EQUIPMENT CHANGING THE COURSE OF A WELL BORE measurement of pressure, flow, liquid, gas and steam MEASUREMENT OF THE QUANTITY OF LIQUIDS AND GASES MEASUREMENT OF THE FLOW OF LIQUIDS, GASES AND VAPORS LIQUID LEVEL MEASUREMENTS OF LOW YIELD PRODUCTS INFORMATION TECHNOLOGIES IN OIL AND GAS PRODUCTION TESTING OF WELL ELECTRIC HEATERS Research deep-well pumping wells EFFICIENCY STUDY ESP cable capital repairs of wells Complex of equipment type KOS and KOS1 DESIGN OF SCREW ROD PUMP DESIGN OF VALVE UNIT corrosion Cranes. FIXING WELLS KTPN MANIFOLDS Pendulum arrangement Safety measures when preparing acid solutions CALCULATION METHOD OF DRILL STRINGS METHODS OF COMBATING PARAFFIN DEPOSITS IN FLOWWELL WELLS Methods of influencing the near-wellbore zone to increase oil recovery METHODS AND TOOLS FOR MEASURING LIQUID LEVEL OSTEY Methods for studying well sections. METHODS OF INDIRECT PRESSURE MEASUREMENTS METHODS OF SALT REMOVAL MECHANISMS OF MOVEMENT AND ALIGNMENT OF DRILLING UNITS MECHANISMS OF MOVEMENT AND ALIGNMENT MECHANISMS DURING LOADING OPERATIONS DURING DRILLING LOADS AFFECTING ON THE UNIT earth equipment Pumping operation of wells PUMPING AND COMPRESSOR PIPES heterogeneous formation Oil and petroleum products Portal news NEW TECHNOLOGICAL AND TECHNICAL ENSURING ENVIRONMENTAL SAFETY OF PRODUCTION PROCESSES EQUIPMENT FOR GAS LIFT WELLS EQUIPMENT FOR MECHANIZATION OF LOGGING OPERATIONS Equipment for oil and gas EQUIPMENT FOR SIMULTANEOUS SEPARATE OPERATION EQUIPMENT FOR PROVIDING OPEN BACKGROUND TANOV GENERAL PURPOSE EQUIPMENT Wellbore equipment completed by drilling COMPRESSOR WELLHEAD EQUIPMENT WELLHEAD EQUIPMENT Wellhead equipment for operation of ESP EQUIPMENT FOR FLOW WELLS EQUIPMENT FOR FLOW WELLS treatment of the bottomhole zone FORMATION OF HYDRATES AND METHODS OF COMBATING THEM FORMATION OF CRYSTAL HYDRATES IN OIL WELLS GENERAL CONCEPTS ABOUT UNDERGROUND AND CAPITAL REPAIRS GENERAL CONCEPTS TIYA ON CONSTRUCTION OF WELLS LIMITING THE INFLOW OF FORMED WATER Hazardous and harmful physical factors DETERMINING PRESSURE AT THE PUMP OUTLET TESTING PROMISING HORIZONS OPTIMIZATION OF SPU OPERATING MODE EXPERIENCE IN OPERATING BORTH WITH A FLEXIBLE TRACTION ELEMENT DEVELOPMENT AND TESTING OF WELLS DEVELOPMENT AND COMMISSIONING OF FLOWER WELLS COMPLICATIONS IN THE PROCESS OF WELL DEEPENING BASIC CONCEPTS AND PROVISIONS BASIC CONCEPTS AND PROVISIONS BASIC INFORMATION ABOUT OIL, GAS AND GAS CONDENSATE FUNDAMENTALS OF HYDRAULIC CALCULATIONS IN DRILLING FUNDAMENTALS OF OIL AND GAS PRODUCTION FUNDAMENTALS OF DIRECTED WELL DESIGN FUNDAMENTALS OF INDUSTRIAL SAFETY CLEANING A DRILLING WELL FROM SLUDGE CLEANING OF ASSOCIATED GAS soldering and surfacing HYDROMECHANICAL DOUBLE-CUP PACKER PGMD1 HYDROMECHANICAL PACKERS SKY, HYDRAULIC AND MECHANICAL PACKERS FOR TESTING COLUMNS PRMP-1 RUBBER-METAL FLOOR PACKERS PACKERS AND ANCHORS PARAMETERS AND COMPLETENESS OF CIRCULATION SYSTEMS Parameters of traveling blocks for working with ASP PRIMARY OPENING OF PRODUCTIVE FORMS PRIMARY METHODS OF CEMENTING MOBILE PUMPING UNITS AND UNITS PROCESSING OF TRAP OIL (OIL SLUDGE) PERIODIC GAS LIFT PROSPECTS FOR USING D WELL INCREASED EFFICIENCY INCREASED OPERATION EFFICIENCY OF SPULS Immersion of pumps under dynamic level Underground equipment of flowing wells LIFTING OF VISCOUS LIQUID THROUGH THE ANNUAL SPACE OF A WELL ROCK DESTRUCTION TOOLS PISTON PRESSURE GAUGES Pressure loss during fluid movement along the tubing Safety rules for well operation Rules for repair work in wells RD 153-39-023-97 PREVENTION OF SALT FORMATION WARNING EXPECTATIONS FOR EDUCATION ASPO WARNINGS FOR EDUCATION ASPO when operating SRP ADVANTAGES OF LONG STROKE Preparation of acid solutions. PREPARATION, CLEANING OF DRILLING MUDS APPLICATION OF JET COMPRESSORS FOR DISPOSAL APPLICATION OF ESP IN WELLS OF OJSC "ORENBURGNEFT" PRINCIPLE OF OPERATION AND DESIGN FEATURES OF BORTH WITH LMP CAUSES AND ANALYSIS OF ACCIDENTS PREDICTION OF SEDIMENTS DURING PRODUCTION CHE OIL DESIGNING THE TRAJECTORY OF DIRECTED WELLS DESIGN, CONSTRUCTION AND ANALYSIS OF HYDROCARBON FIELDS DEVELOPMENT Pump performance WELL WASHING AND DRILLING FLUIDS FIELD RESEARCH FIELD METHODS FOR DETERMINING NOSE FORMATION ZONES FIELD COLLECTION AND PREPARATION OF OIL, GAS AND WATER BLOCKOUT CONTROL EQUIPMENT WAYS TO INCREASE WELL OPERATION EFFICIENCY BUILDING OF PRODUCTION AND INJECTION WELLS FOR MISCELLANEOUS DESTRUCTION OF ROCKS DISTRIBUTION OF BREAKS ALONG THE LENGTH OF ROD COLUMNS CALCULATION OF BOTTOM CALCULATION OF BOTTOM PRODUCTIVITY Regulating the properties of cement mortar and stone using reagents Modes of production and injection wells. RESERVES FOR REDUCING ENERGY CONSUMPTION DURING OPERATION REPAIRS FOR ENVIRONMENTAL IMPROVEMENT OF THE WELL FACILITIES ROLE OF FLOWER PIPES SELF-PROPELLED UNITS WITH MOBILE... WELL LOCATION GRID LIGHT HYDROCARBONS COLLECTION SYSTEMS Downhole seals (packers) Downhole centrifugal pumps for oil production COMPOSITION AND SOME PROPERTIES OF WATER IN OIL AND GAS PLACES SPECIAL NON-INSERTED ROD PUMP OIL PRODUCTION METHODS APPLIED AT OJSC FIELDS METHODS OF ASSESSING THE CONDITION OF POP CONDITION COMPARATIVE TESTS OF PUMPING UNITS MEANS AND METHODS FOR CHECKING GAS QUANTITY COUNTERS MEANS AND METHODS FOR CHECKING LIQUID QUANTITY METERS STAGES OF FIELDS DEVELOPMENT Pumping machines Jet pumps jet pump GAS QUANTITY METERS LIQUID QUANTITY METERS Traveling MECHANISMS TEMPERATURE AND PRESSURE IN ROCKS AND WELLS Theoretical foundations safety FLOW MEASUREMENT TECHNIQUES Technical physics TRAJECTORY OF MOVEMENT OF THE BOTTOM OF A WELL Pipes INSTRUCTIONS FOR CALCULATING SHORT CIRCUIT CURRENTS CONDITIONS OF LIQUID AND GAS INFLOW INTO WELLS Installations of hydraulic piston pumps for oil production Installations of submersible screw electric pumps Installations of submersible diaphragms ragmen electric pumps Wellhead equipment WEIGHTED DRILL PIPES ESP ESP completely FACTORS AFFECTING INTENSITY OF FORMATION OF ASPO Physical and mechanical properties of reservoir rocks PHYSICAL CHARACTERISTICS OF GASES OF OIL AND GAS PLACES FILTERS Fountain method of oil production CEMENTING OF WELLS CIRCULATION SYSTEMS OF DRILLING RIGS Slag-sand cements Slag-sand cements of joint grinding Sucker rods (SR) ROD PUMPING UNITS (SRPU) ROD PUMPS FOR LISCAL LIFTING OIL ROD WELL PUMPS Rod well pumps SSN OPERATION OF GAS WELLS operation of low-yield wells OPERATION OF LOW-yield wells IN CONTINUOUS MODE OPERATION OF WATERED PARAFFIN-CONTAINING WELLS WELL TATION OPERATION OF WELLS ESP ELECTRIC DEHYDRATOR. ELECTRIC DIAPHRAGM PUMP energy saving downhole electric pump unit YAKORILet's talk about how toluene nitration is carried out. Through such interaction, a huge number of semi-finished products used in the manufacture are obtained. explosives, pharmaceuticals.
Importance of nitration
Benzene derivatives in the form of aromatic nitro compounds are produced in the modern chemical industry. Nitrobenzene is an intermediate product in aniline dye, perfume, and pharmaceutical production. It is an excellent solvent for many organic compounds, including cellulose nitrite, forming a gelatinous mass with it. In the petroleum industry it is used as a purifier for lubricating oils. The nitration of toluene produces benzidine, aniline, and phenylenediamine.
Nitration Characteristics
Nitration is characterized by the introduction of a NO2 group into the molecule organic compound. Depending on the starting substance, this process occurs according to a radical, nucleophilic, or electrophilic mechanism. Nitronium cations, NO2 ions and radicals act as active particles. The nitration reaction of toluene is a substitution reaction. For others organic matter Substitutive nitration, as well as addition at a double bond, is possible.
Nitration of toluene in an aromatic hydrocarbon molecule is carried out using a nitrating mixture (sulfuric and nitric acids). It acts as a water-removing agent in this process and exhibits catalytic properties.

Process equation
Nitration of toluene involves the replacement of one hydrogen atom with a nitro group. What does the flow diagram of the process look like?
In order to describe the nitration of toluene, the reaction equation can be represented as follows:
ArH + HONO2+ = Ar-NO2 +H2O
It allows one to judge only the general course of interaction, but does not reveal all the features of this process. What actually occurs is a reaction between aromatic hydrocarbons and nitric acid products.
Considering that the products contain water molecules, this leads to a decrease in the concentration of nitric acid, so the nitration of toluene slows down. In order to avoid this problem, this process is carried out at low temperatures, using nitric acid in excess quantities.
In addition to sulfuric acid, polyphosphoric acids and boron trifluoride are used as water-removing agents. They make it possible to reduce the consumption of nitric acid and increase the efficiency of interaction.

Process nuances
Nitration of toluene was described at the end of the nineteenth century by V. Markovnikov. He was able to establish a connection between the presence in the reaction mixture and the rate of the process. In modern production of nitrotoluene, anhydrous nitric acid is used, taken in some excess.
In addition, the sulfonation and nitration of toluene involves the use of the available water-removing component boron fluoride. Its introduction into the reaction process makes it possible to reduce the cost of the resulting product, which makes the nitration of toluene accessible. Equation of the ongoing process in general view presented below:
ArH + HNO3 + BF3= Ar-NO2 + BF3 ·H2 O
After completion of the reaction, water is introduced, due to which boron fluoride monohydrate forms a dihydrate. It is distilled off in a vacuum, then calcium fluoride is added, returning the compound to its original form.

Specifics of nitration
There are some features of this process related to the choice of reagents and reaction substrate. Let's look at some of their options in more detail:
- 60-65 percent nitric acid mixed with 96 percent sulfuric acid;
- a mixture of 98% nitric acid and concentrated sulfuric acid is suitable for slightly reactive organic substances;
- Potassium or ammonium nitrate with concentrated sulfuric acid is an excellent choice for the production of polymer nitro compounds.

Nitration kinetics
Reacting with a mixture of sulfuric and nitric acids, they are nitrated by an ionic mechanism. V. Markovnikov managed to characterize the specifics of this interaction. The process takes place in several stages. First, nitrosulfuric acid is formed, which undergoes dissociation in an aqueous solution. Nitronium ions react with toluene, forming nitrotoluene as a product. When water molecules are added to the mixture, the process slows down.
In solvents with an organic nature - nitromethane, acetonitrile, sulfolane - the formation of this cation allows you to increase the rate of nitration.
The resulting nitronium cation attaches to the aromatic toluene core, forming an intermediate. Next, proton abstraction occurs, leading to the formation of nitrotoluene.
For detailed description of the ongoing process, one can consider the formation of “sigma” and “pi” complexes. The formation of the “sigma” complex is the limiting stage of the interaction. will be directly related to the rate of addition of the nitronium cation to the carbon atom in the nucleus of the aromatic compound. The removal of a proton from toluene occurs almost instantly.
Only in certain situations may there be any substitution problems associated with a significant primary kinetic isotope effect. This is due to acceleration reverse process in the presence of various types of obstacles.
When choosing concentrated sulfuric acid as a catalyst and water-removing agent, a shift in the equilibrium of the process towards the formation of reaction products is observed.

Conclusion
The nitration of toluene produces nitrotoluene, which is a valuable product of the chemical industry. This substance is an explosive compound, therefore it is in demand in blasting operations. Among environmental problems associated with its industrial production, we note the use of a significant amount of concentrated sulfuric acid.
To cope with this problem, chemists are looking for ways to reduce the sulfuric acid waste produced after the nitration process. For example, the process is carried out at low temperatures and easily regenerated media are used. Sulfuric acid has strong oxidizing properties, which negatively affects the corrosion of metals and poses an increased danger to living organisms. If you comply with all safety standards, you can cope with these problems and obtain high-quality nitro compounds.
a) Nitration of benzene (traction!). In a small flask, mix 4 ml of concentrated sulfuric acid ( = 1.84) with 3 ml of concentrated nitric acid ( = 1.4). 3 ml of benzene is added dropwise to the resulting mixture, shaking the contents of the flask vigorously (the temperature should not rise above 40 C), cooling with water if necessary. Having closed the flask with a stopper with an air cooler, heat it for 15 minutes in a water bath to 60 0 C, shaking frequently. The reaction mixture is then cooled and poured into a glass with 20 ml of ice water, thereby forming two layers. The aqueous layer is drained, and the oil (nitrobenzene) that has precipitated at the bottom is washed twice more with water. After separation from water, crude nitrobenzene is poured into a dry test tube, 2-3 pieces of calcined CaCl2 are added and heated in a water bath until the nitrobenzene becomes transparent. Nitrobenzene is distilled from a small Wurtz flask or test tube with a downward tube at 207-210 0 C. (Nitrobenzene cannot be distilled to dryness! An explosion is possible!).
Write the equation for the nitration of benzene. What is the role of sulfuric acid in the nitration of aromatic compounds? Explain the mechanism of nitration of aromatic compounds.
c) Nitration of toluene. When toluene is nitrated, a mixture may form ortho- And pair- nitrotoluenes. Monitoring the process and identifying reaction products can be carried out using thin layer chromatography. Chromatography is carried out on a silufol plate using carbon tetrachloride as an eluent. Prepare a nitrating mixture of 3 ml of concentrated nitric acid ( = 1.1) and 1 ml of concentrated sulfuric acid. The nitrating mixture is added dropwise into a test tube with 2 ml of toluene while cooling and shaking the reaction mixture. Then the test tube is closed with a stopper with a vertical tube and heated in a water bath, shaking frequently. After 10 minutes, a sample of the reaction mass is taken with a capillary and a sample of the solution and “witnesses” are applied to the starting line of the silufol plate. ortho - And pair- nitrotoluenes (in toluene). The plate is lowered into a chamber containing carbon tetrachloride and the appearance of nitrotoluenes is noted.
The next sample of the solution is taken after 40 minutes, and the third - after 1 hour. The change in the composition of the reaction medium is noted.
Nitration
The nitration reaction is one of the most studied reactions aromatic substitution. For preparative purposes, nitration is usually carried out with a mixture of concentrated nitric and sulfuric acids, the so-called nitrating mixture . At the first stage of the reaction, the formation of nitronium ion + NO 2 occurs, which is an electrophilic agent:
HO-NO 2 + H 2 SO 4 H 2 O + -NO 2 + HSO 4 -
H 2 O + -NO 2 + H 2 SO 4 H 3 O + + HSO 4 - + + NO 2
The presence of nitronium ion in this solution was confirmed spectroscopically. Nitric acid in concentrated sulfuric acid is almost completely converted into a nitronium cation. The insignificant effectiveness of nitric acid itself in the nitration reaction of benzene is explained by the low content of the + NO 2 ion.
Other systems are also used as nitrating agents, in which either a cation + NO 2 or a compound of the general formula NO2-Y is generated where Y is a good leaving group. Some of these systems that have found the greatest application are presented in Table 1 in order of increasing activity.
Table 1. Nitrating reagents.
| Nitrating reagent | Generation method | Arenas subject to nitration |
| Nitric acid HO-NO2 |
Phenols, phenol ethers, biphenyl | |
| Acetyl nitrate CH 3 C(O)-O-NO 2 | CH 3 COOH + HNO 3 (CH 3 CO) 2 O + HNO 3 | Benzene, alkylbenzenes |
| Nitrogen dioxide N 2 O 4 (O=N-O-NO 2) |
Benzene, alkylbenzenes | |
| Nitrating mixture | H 2 SO 4 conc + HNO 3 | Benzene, alkylbenzenes, halobenzenes, benzoic acid, nitrobenzene, naphthalene |
| Nitronium chloride Сl-NO 2 |
Benzene, alkylbenzenes, nitrobenzene, | |
| Nitronium tetrafluoroborate BF 4 -+ NO 2 | HF. 2BF 3 + HNO 3 | Dinitrobenzene |
Using the example of the nitration reaction of alkylbenzenes, the influence of spatial factors on the direction of electrophilic substitution is clearly visible. Thus, during the nitration of toluene (methylbenzene) ortho-isomer is formed as the main product, and upon transition to ethyl-, iso-drank- and especially to rubs-butyl-benzene, its yield decreases significantly (see Table 2).
Table 2. Influence of spatial factors on the ratio of ortho-, para-isomers in the nitration reaction (NO 2 +)
Substitution in positions, % |
|||
C6H5 -C2H5 |
|||
C6H5-CH(CH3)2 |
|||
C6H5-C(CH3)3 |
|||
When studying the nitration of alkylbenzenes, the so-called ipso-substitution , when an electrophilic attack occurs at the carbon atom of the benzene ring that already contains a substituent, for example:

Unlike nitration, during halogenation the attack of the aromatic substrate can be carried out by various electrophiles. Free halogens, for example, Cl 2 and Br 2 (note 35) can easily attack the activated aromatic ring (for example, phenol), but are not able to react with benzenes and alkylbenzenes (photochemical activation can, however, in the latter case lead to the occurrence of radical side chain substitutions; see section IV.3). To polarize the attacking halogen molecule, it is necessary Lewis acid catalysis such as AlCl 3, FeBr 3, etc.; in this case, the so-called “electrophilic end” appears in the halogen molecule (the energy required for the formation of the Hal + cation is significantly higher). This makes electrophilic substitution much easier:

Halogenation proceeds very vigorously if reagents are used in which the halogen, as a result of polarization, has a strong positive charge or even exists as a cation. Yes, very inert meta-dinitrobenzene can be brominated with bromine in concentrated sulfuric acid in the presence of silver sulfate. It is assumed that in this case a bromine cation is formed intermediately:
2Br 2 + Ag 2 SO 4 2Br + + 2AgBr + SO 4 2-
Reactivity elemental iodine in electrophilic substitution reactions in the aromatic ring is insignificant, so direct iodination is possible only in the case of phenol and aromatic amines. Iodination of other aromatic compounds is carried out in the presence of an oxidizing agent (usually nitric acid). It is believed that under these conditions the role of the electrophilic agent is played by the I- + OH 2 ion.

For the halogenation of arenes, it can also be used mixed halogens, for example, bromine monochloride (BrCl) or iodine (ICl):


Halogenation in vivo. As an example of electrophilic aromatic halogenation occurring in living organisms, we can cite the reaction of iodination of the amino acid tyrosine during the biosynthesis of iodine-containing thyroid hormones to 3-iodotyrosine and then to 3,5-diiodotyrosine:

The details of the mechanism of sulfonation have been studied in less detail compared to nitration and halogenation. Benzene itself is sulfonated rather slowly with hot concentrated sulfuric acid, but quickly with oleum, SO 3 in inert solvents, or a complex of SO 3 with pyridine. The nature of the electrophilic species depends on the reaction conditions, but it is probably always SO 3, either in a free state or bound to a “carrier”, for example, in the form of H 2 SO 4. SO 3 (H 2 S 2 O 7) in sulfuric acid. Small amounts of SO 3 are formed in H 2 SO 4:
2H 2 SO 4 SO 3 + H 3 O + + HSO 4 -
The attack of the aromatic substrate is carried out by the sulfur atom since it is strongly positively polarized, that is, electron-deficient:

Sulfonation is reversible process. It has practical significance: when treating sulfonic acids with steam, the SO 3 H group is replaced by hydrogen. Thus, it is possible to introduce the SO 3 H group as a substituent that orients subsequent reactions in the required manner (see section IV.1.B), and then remove it. The sulfonation of naphthalene has some interesting features (see section IV.1.D).
Like halogens, alkyl halides can be so highly polarized Lewis acids(aluminum and zinc chlorides, boron trifluoride, etc.) that they become capable of electrophilic substitution in the aromatic ring:
R-Cl + AlCl 3 R +... Cl ...- AlCl 3 R + AlCl 4 -
In addition to alkyl halides, alkenes or alcohols can be sources of carbocations for the halogenation of aromatic compounds. In this case, the presence of a protic acid is necessary to protonate the alkene or alcohol. In the case of alcohols, an additive is required no less than equimolar amount of acid (since the water released during the reaction deactivates an equimolar amount of the catalyst), whereas in reactions involving alkyl halides and alkenes it is sufficient to add a small amount of catalyst.
In the laboratory, Friedel-Crafts alkylation has limited use, since this reaction usually produces mixtures of products, due to a number of reasons:
1) The resulting alkylation product undergoes electrophilic aromatic substitution reactions more easily than the starting compound (Alk is an electron-donating group), therefore the product is then preferentially alkylated. If one wants to obtain monoalkylation products, then it is necessary to take a large excess of the aromatic compound.
2) Like sulfonation, the Friedel-Crafts alkylation reaction reversible(see also section IV.1.D).
3) Even under mild conditions, primary and secondary alkyl halides give preferentially secondary or tertiary alkylarenes, respectively, since alkylation occurs under conditions approaching S N 1 reactions. (note 37) Regrouping can be avoided if you work at low temperatures.
The Friedel-Crafts acylation of aromatic compounds is the most important method for the synthesis of fatty aromatic ketones. Carboxylic acid derivatives, such as acyl halides and anhydrides, have a polar carbonyl group and are in principle capable of electrophilic substitution in aromatic systems:

The electrophilic activity of these compounds, however, is low and must be enhanced by the action of Lewis acids. In this case, the acid catalyst, as a rule, attacks the oxygen atom carbonyl compound and, by shifting the electron density, increases the positive charge of the neighboring carbon atom. As a result, a polarized complex is formed (and, in the limit, an acyl cation), acting as an electrophile:

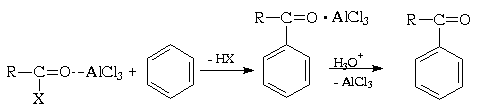
An important difference between the acylation reaction with acyl halides and the alkylation reaction with alkyl halides is that in the first of these reactions more than 1 mole Lewis acid required, while in the second only catalytic amounts are needed. This is due to the fact that the Lewis acid forms a complex with both the acylating derivative of the carboxylic acid and the ketone, the reaction product. When reacting with anhydrides, the resulting acid binds another mole of catalyst, so that in total at least two moles are needed. In each case, upon completion of the reaction, the resulting complex of the ketone with aluminum chloride (or other Lewis acid) must be hydrolytically destroyed ( hydrochloric acid with ice).
No polyacylation is observed because the resulting ketone is significantly less reactive than the parent compound (see section IV.1.B). Therefore, it is often preferred to obtain alkylbenzenes not by direct alkylation, but by Friedel-Crafts acylation followed by reduction. Aromatic compounds with strongly deactivating substituents, such as nitro or cyano groups, are also not Friedel-Crafts acylated.
Control tasks
2. Draw a potential energy diagram for an electrophilic aromatic substitution reaction in which the slow step is the formation
-complex (for example, nitration of benzene with nitronium borofluoride;
see section IV.1.A).
3. Which product is predominantly formed during bromination: a) pair-nitrotoluene; b) meta-nitrobenzenesulfonic acids; V) ortho-nitrophenol.
4. Adrenaline (1-(3,4"-dihydroxyphenyl)-2-methylaminoethanol) is the first hormone isolated from the adrenal medulla; it is currently synthesized in three stages from pyrocatechol. Write the equation for the first stage of this synthesis - the reaction of acylation of pyrocatechol (1,2-dihydroxybenzene) with chloroacetic acid chloride and explain the mechanism).
5. One of them qualitative reactions to proteins is a xanthoprotein reaction, indicating the presence of aromatic amino acids. It involves processing protein nitric acid when heated. Write the equation for the xanthoprotein reaction with tyrosine (see Section I), formed as a result of protein hydrolysis.
Physical properties
Benzene and its closest homologues are colorless liquids with a specific odor. Aromatic hydrocarbons are lighter than water and do not dissolve in it, but they are easily soluble in organic solvents - alcohol, ether, acetone.
Benzene and its homologues are themselves good solvents for many organic substances. All arenas burn with a smoky flame due to the high carbon content in their molecules.
The physical properties of some arenas are presented in the table.
Table. Physical properties of some arenas
|
Name |
Formula |
t°.pl., |
t°.b.p., |
|
Benzene |
C6H6 |
5,5 |
80,1 |
|
Toluene (methylbenzene) |
C 6 H 5 CH 3 |
95,0 |
110,6 |
|
Ethylbenzene |
C 6 H 5 C 2 H 5 |
95,0 |
136,2 |
|
Xylene (dimethylbenzene) |
C 6 H 4 (CH 3) 2 |
||
|
ortho- |
25,18 |
144,41 |
|
|
meta- |
47,87 |
139,10 |
|
|
pair- |
13,26 |
138,35 |
|
|
Propylbenzene |
C 6 H 5 (CH 2) 2 CH 3 |
99,0 |
159,20 |
|
Cumene (isopropylbenzene) |
C 6 H 5 CH(CH 3) 2 |
96,0 |
152,39 |
|
Styrene (vinylbenzene) |
C 6 H 5 CH=CH 2 |
30,6 |
145,2 |
Benzene – low boiling ( tbale= 80.1°C), colorless liquid, insoluble in water
Attention! Benzene – poison, affects the kidneys, changes the blood formula (with prolonged exposure), can disrupt the structure of chromosomes.
Majority aromatic hydrocarbons life-threatening, toxic.
Preparation of arenes (benzene and its homologues)
In the laboratory
1. Fusion of benzoic acid salts with solid alkalis
C6H5-COONa + NaOH t → C 6 H 6 + Na 2 CO 3
sodium benzoate
2. Wurtz-Fitting reaction: (here G is halogen)
C 6H 5 -G + 2Na + R-G →C 6 H 5 - R + 2 NaG
WITH 6 H 5 -Cl + 2Na + CH 3 -Cl → C 6 H 5 -CH 3 + 2NaCl
In industry
- isolated from oil and coal by fractional distillation and reforming;
- from coal tar and coke oven gas
1. Dehydrocyclization of alkanes with more than 6 carbon atoms:
C6H14 t , kat→C 6 H 6 + 4H 2
2. Trimerization of acetylene(for benzene only) – r. Zelinsky:
3С 2 H 2 600°C, act. coal→C 6 H 6
3. Dehydrogenation cyclohexane and its homologues:
Soviet academician Nikolai Dmitrievich Zelinsky established that benzene is formed from cyclohexane (dehydrogenation of cycloalkanes
C6H12 t, kat→C 6 H 6 + 3H 2

C6H11-CH3 t , kat→C 6 H 5 -CH 3 + 3H 2
methylcyclohexantoluene
4. Alkylation of benzene(preparation of benzene homologues) – r Friedel-Crafts.
C 6 H 6 + C 2 H 5 -Cl t, AlCl3→C 6 H 5 -C 2 H 5 + HCl
chloroethane ethylbenzene

Chemical properties of arenes
I. OXIDATION REACTIONS
1. Combustion (smoking flame):
2C6H6 + 15O2 t→12CO 2 + 6H 2 O + Q
2. Under normal conditions, benzene does not discolor bromine water and aqueous solution potassium permanganate
3. Benzene homologues are oxidized by potassium permanganate (discolor potassium permanganate):
A) in an acidic environment to benzoic acid
When benzene homologues are exposed to potassium permanganate and other strong oxidizing agents, the side chains are oxidized. No matter how complex the chain of the substituent is, it is destroyed, with the exception of the a-carbon atom, which is oxidized into a carboxyl group.
Homologues of benzene with one side chain give benzoic acid:
Homologues containing two side chains give dibasic acids:
5C 6 H 5 -C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 → 5C 6 H 5 COOH + 5CO 2 + 6K 2 SO 4 + 12MnSO 4 +28H 2 O
5C 6 H 5 -CH 3 + 6KMnO 4 + 9H 2 SO 4 → 5C 6 H 5 COOH + 3K 2 SO 4 + 6MnSO 4 +14H 2 O
Simplified :
C6H5-CH3+3O KMnO4→C 6 H 5 COOH + H 2 O
B) in neutral and slightly alkaline to benzoic acid salts
C 6 H 5 -CH 3 + 2KMnO 4 → C 6 H 5 COO K + K OH + 2MnO 2 + H 2 O
II. ADDITION REACTIONS (harder than alkenes)
1. Halogenation
C 6 H 6 +3Cl 2 h ν → C6H6Cl6 (hexachlorocyclohexane - hexachlorane)
2. Hydrogenation
C6H6 + 3H2 t , PtorNi→C 6 H 12 (cyclohexane)
3. Polymerization
III. SUBSTITUTION REACTIONS – ion mechanism (lighter than alkanes)
1. Halogenation -
a ) benzene
C6H6+Cl2 AlCl 3 → C 6 H 5 -Cl + HCl (chlorobenzene)
C6H6 + 6Cl2 t ,AlCl3→C 6 Cl 6 + 6HCl( hexachlorobenzene)
C 6 H 6 + Br 2 t,FeCl3→ C 6 H 5 -Br + HBr( bromobenzene)
b) benzene homologues upon irradiation or heating
By chemical properties alkyl radicals are similar to alkanes. The hydrogen atoms in them are replaced by halogen by a free radical mechanism. Therefore, in the absence of a catalyst, upon heating or UV irradiation, a radical substitution reaction occurs in the side chain. The influence of the benzene ring on alkyl substituents leads to the fact that the hydrogen atom is always replaced by the carbon atom directly bonded to benzene ring(a-carbon atom).
1) C 6 H 5 -CH 3 + Cl 2 h ν → C 6 H 5 -CH 2 -Cl + HCl
c) benzene homologues in the presence of a catalyst
C 6 H 5 -CH 3 + Cl 2 AlCl 3 → (mixture of orth, pair of derivatives) +HCl
2. Nitration (with nitric acid)
C 6 H 6 + HO-NO 2 t, H2SO4→C 6 H 5 -NO 2 + H 2 O
nitrobenzene - smell almonds!
C 6 H 5 -CH 3 + 3HO-NO 2 t, H2SO4→ WITH H 3 -C 6 H 2 (NO 2) 3 + 3H 2 O2,4,6-trinitrotoluene (tol, TNT)
Application of benzene and its homologues
Benzene C 6 H 6 is a good solvent. Benzene as an additive improves the quality of motor fuel. It serves as a raw material for the production of many aromatic organic compounds - nitrobenzene C 6 H 5 NO 2 (solvent from which aniline is obtained), chlorobenzene C 6 H 5 Cl, phenol C 6 H 5 OH, styrene, etc.
Toluene C 6 H 5 –CH 3 – solvent, used in the production of dyes, medicinal and explosives (TNT (TNT), or 2,4,6-trinitrotoluene TNT).
Xylenes C6H4(CH3)2. Technical xylene is a mixture of three isomers ( ortho-, meta- And pair-xylenes) – used as a solvent and starting product for the synthesis of many organic compounds.
Isopropylbenzene C 6 H 5 –CH(CH 3) 2 is used to produce phenol and acetone.
Chlorinated derivatives of benzene used for plant protection. Thus, the product of the replacement of H atoms in benzene with chlorine atoms is hexachlorobenzene C 6 Cl 6 - a fungicide; it is used for dry treatment of wheat and rye seeds against smut. The product of the addition of chlorine to benzene is hexachlorocyclohexane (hexachlorane) C 6 H 6 Cl 6 - an insecticide; it is used to control harmful insects. The substances mentioned belong to pesticides - chemical means of combating microorganisms, plants and animals.
Styrene C 6 H 5 – CH = CH 2 very easily polymerizes, forming polystyrene, and when copolymerizing with butadiene, styrene-butadiene rubbers.
VIDEO EXPERIENCES

 Entrance
Entrance